
The 1s orbital and 2s orbital both have the characteristics of an s orbital (radial nodes, spherical volume probabilities, can only hold two electrons, etc.) but, as they are found in different energy levels, they occupy different spaces around the nucleus. Orbitals on different energy levels are similar to each other, but they occupy different areas in space. The energy level is determined by the period and the number of electrons is given by the atomic number of the element.
:max_bytes(150000):strip_icc()/carbonatom-58b602855f9b5860464c8bf6.jpg)
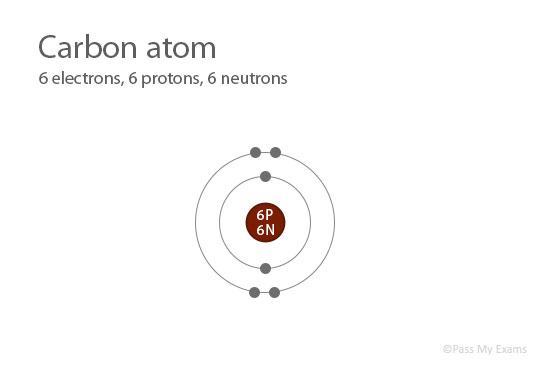
The p, d, and f orbitals have different sublevels, thus can hold more electrons.Īs stated, the electron configuration of each element is unique to its position on the periodic table. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within 95% probability. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons. The elements placed in group 18 are called the noble gas electrons which have completely filled configurations and are highly stable and unreactive.\)īefore assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Note: We should know the atomic number of the elements, so that we could write the electronic configuration of the atom and we should be thorough with the order of orbitals and number of electrons each shell can accommodate to write the configuration of atoms. Now let’s write the electronic configuration of carbon as, The carbon atom belongs to the second period of the periodic table. We know that the atomic number of carbon is 6.Therefore there will be 6 electrons present in its o-orbitals. Let’s solve the problem and get an idea and difference between the two types of representation of electrons in carbon. It is quite confusing to explain with the aid of words. The electronic configuration is the representation of the total number of electrons in each orbitals of the atom in the increasing order of their energy.īut in noble gas electronic configuration, we will insert the chemical notation of the noble gas which is present in the previous period and write the further electrons present other than those present in the orbitals of the noble gas.

To solve this problem we should know the difference between the electronic configuration and noble gas electronic configuration of an atom. Now in the question we are supposed to write the noble gas electronic configuration. And the atomic number of the element gives the number of electrons and protons in the atom whereas the atomic mass number is the sum total of the number of protons and neutrons in the atom. We know that the elements are placed in periodic take in accordance with the increasing order of the periodic table. From the lower classes we are very familiar with the elements and its atomic number and positioning in the periodic table. In the question it is asked how will be the noble gas configuration of a carbon atom. Trace the noble gas present in the previous period with respect to carbon.


 0 kommentar(er)
0 kommentar(er)
